Catalogue
FIX&PERM® Sample Kit
Catalog number: GAS-002M$100.00
Add To Cart| Product Type |
Buffers and Reagents |
| Units | 2 x 5ml |
| Application |
Flow Cytometry |
Background
Flow cytometric analyses with monoclonal antibodies were so far mainly restricted to cell surface molecules. Intracellular structures such as cytoplasmic or nuclear enzymes, oncoproteins, cytokines, immunoglobulins etc. were largely excluded from such studies. Also excluded from flow cytometric studies were cytoplasmic localizations of well-established membrane molecules like CD3 and CD22, which, in their cytoplasmic form, are the most reliable lineage markers in undifferentiated leukemia. With the FIX&PERM® Kit flow cytometric analysis of intracellular antigens has become as easy as surface antigen studies. The only prerequisite is the availability of suitable antibody conjugates. Most of the available monoclonal antibody conjugates can be used with the FIX&PERM® Kit, some determinants are sensitive, however, to the fixation step involved. This and the optimal fixation time have to be tested for each reagent.
Results must be interpreted by a certified professional before final interpretation. Analyses performed with this antibody should be paralleled by positive and negative controls. If unexpected results are obtained which cannot be attributed to differences in laboratory procedures, please contact us.
Product
This FIX&PERM® Cell Fixation and Permeabilization Kit contains 2 reagents: Fixation Medium (Reagent A) and Permeabilization Medium (Reagent B). It is intended for first fixing cells in suspension with Reagent A and then permeabilizing the cell membranes with Reagent B. This procedure gives antibodies access to intracellular structures and leaves the morphological scatter characteristics of cells intact. Specific formulations reduce background staining and allow simultaneous addition of permeabilization medium and fluorochrome labeled antibodies. FIX&PERM® is suitable for the analysis of normal and malignant leukocyte populations derived from various human biological samples (blood, bone marrow and others) using flow cytometry. Results must be put within the context of other diagnostic tests as well as the clinical history of the patient by a certified professional before final interpretation.
Format: 1 x 5ml vial of Fixation Medium (Reagent A) and 1 x 5ml vial Permeabilization Medium (Reagent B).
FIX&PERM® Reagents are designed for use with all commercially available flow cytometers. Alignment and compensation should be performed according to manufacturer´s instructions.
The quality of each FIX&PERM® Lot is determined by fixation and permeabilization of well defined blood samples from representative donors and subsequent comparison of forward and side scatter characteristics of obtained leukocytes, as well as immunostaining efficiency for several membranous and cytoplasmic antigens
Applications
Biological fluids (blood, bone marrow, and others) must be collected under sterile conditions. Anticoagulation with EDTA or heparin is recommended. The samples should be stored at room temperature until used. For optimal results, samples should be processed and analyzed within 24 hours. Samples with high numbers of non-viable cells might cause false results, such cases require determination of cell viability with e.g. propidium iodide. All biological samples have to be handled with caution. Always consider them as potentially infective. Use appropriate precautions such as gloves, lab-coat, etc.
Fixation, permeabilization and staining procedure:
• For each sample to be analyzed add 50 µl of whole blood, bone marrow or mononuclear cell suspension in a 5ml tube
• Add 100 µl of Reagent A (Fixation Medium, stored and used at room temperature)
• Incubate for 15 minutes at room temperature
• Add 5ml phosphate buffered saline and centrifuge cells for 5 minutes at 300 g
• Remove supernatant and add to cell pellet 100 µl Reagent B (Permeabilization Medium) and 20 µl of the appropriate Nordic-MUbio monoclonal antibody conjugate
• Vortex at low speed for 1-2 seconds
• Incubate for 15 minutes at room temperature
• Wash cells with phosphate buffered saline as described above
• Remove supernatant and resuspend cells in sheath fluid for immediate analysis or resuspend cells in 0.5 ml 1.0 % formaldehyde and store them at 2-8°C in the dark. Analyze fixed cells within 24 hours.
Comments: Special cases (diluted bone marrow samples, other samples containing low soluble protein) might benefit from replenishment with plasma components before the FIX&PERM® treatment in order to create a milieu, which more closely resembles the stuation in anti-coagulated blood. For that pupose addition of IgG preparations (e.g. Beriglobulin P, ZLB Behring, final concentration 10mg/ml) and human serum albumin (e.g. human albumin “Behring” 20% - infusion solution, final concentration 40mg/ml) is recommended.
Limitations of the technique: Flow cytometry should be performed by professional users only. Improper alignment of the flow cytometer, inaccurate compensation of fluorescence leaking into other channels as well as incorrect positioning of regions may lead to false results. Lysis of red cells might be impossible for various reasons. In such instances it is recommended to isolate mononuclear cells (MNC) via density gradient centrifugation prior to staining. Results will be correct and reproducible as long as the procedures used respect the technical recommendations and obey good laboratory practice. The FIX&PERM® solutions are provided in a concentration that will allow to fix and permeabilize human hematopoietic cells. It is therefore strongly recommended to stick to the working protocol in terms of concentration and volume regarding cells and antibody. The properties of FIX&PERM® have been determined using EDTA anti-coagulated peripheral blood.
Storage
FIX&PERM® Cell Fixation and Permeabilization Kit reagents should be stored and used at room temperature. Do not freeze. Stability of the reagent: Please refer to the expiry date printed onto the vial. The use of the reagent after the expiration date is not recommended. If reagents are stored under any conditions other than those specified, the conditions must be verified by the user. Do not use reagents if a precipitate should form or discoloration occurs.
If unexpected results are obtained which cannot be attributed to differences in laboratory procedures, please contact us.
Shipping Conditions: Ship at ambient temperature
Caution
This product is intended FOR RESEARCH USE ONLY, and FOR TESTS IN VITRO, not for use in diagnostic or therapeutic procedures involving humans or animals.For professional users only. Reagent A of FIX&PERM® Cell Fixation and Permeabilization Kit contains fomaldehyde and is labelled: Harmful. Formaldehyde is toxic, allergenic and a suspected carcinogen. Never pipette by mouth and avoid contact with eyes, skin and clothing. Proper handling procedures are recommended. As a main rule, persons under 18 years of age are not allowed to work with this product. Users must be carefully instructed in the proper working procedure, the dangerous properties of the product and the necessary safety instructions. Please refer to the Safety Data Sheet (SDS) for additional information. Dispose product remainders according to local regulations.
References
Recent application papers:
Mestrum SGC, de Wit NCJ, Drent RJM, Hopman AHN, Ramaekers FCS, Leers MPG.Proliferative activity is disturbed in myeloproliferative neoplasms (MPN), myelodysplastic syndrome (MDS), and MDS/MPN diseases. Differences between MDS and MDS/MPN.
Cytometry B Clin Cytom. 2021 May;100(3):322-330. doi: 10.1002/cyto.b.21946.
Mestrum SGC, Cremers EMP, de Wit NCJ, Drent RJM, Ramaekers FCS, Hopman AHN, Leers MPG. Integration of the Ki-67 proliferation index into the Ogata score improves its diagnostic sensitivity for low-grade myelodysplastic syndromes. Leuk Res. 2022 Feb;113:106789. doi: 10.1016/j.leukres.2022.106789.
Mestrum SGC, Cremers EMP, de Wit NCJ, Drent RJM, Ramaekers FCS, Hopman AHN, Leers MPG. Optimized gating strategy and supporting flow cytometry data for the determination of the Ki-67 proliferation index in the diagnosis of myelodysplastic syndrome. Data Brief. 2022 Feb 22;41:107976. doi: 10.1016/j.dib.2022.107976.
Nies KPH, Kraaijvanger R, Lindelauf KHK, Drent RJMR, Rutten RMJ, Ramaekers FCS, Leers MPG. Determination of the proliferative fractions in differentiating hematopoietic cell lineages of normal bone marrow. Cytometry A. (2018) 93, 1097-1105 . doi: 10.1002/cyto.a.23564
Further reading:
- Gerna, G., Percivalle, E., Lilleri, D., Lozza, L., Fornara, C., Hahn, G., Baldanti, F. & Revello, M. G. (2005) J Gen Virol 86, 275-84.
- Groeneveld, K., te Marvelde, J. G., van den Beemd, M. W., Hooijkaas, H. & van Dongen, J. J. (1996) Leukemia 10, 1383-9.
- Haranaga, S., Yamaguchi, H., Friedman, H., Izumi, S., & Yamamoto, Y. (2001) Infect Immun 69, 7753-9.
- Hegazy, A. N. & Klein, C. (2008) Leukemia 22, 2070-9.
- Kappelmayer, J., Gratama, J. W., Karaszi, E., Menendez, P., Ciudad, J., Rivas, R. & Orfao, A. (2000) J Immunol Methods 242, 53-65.
- Kline, M. P., Rajkumar, S. V., Timm, M. M., Kimlinger, T. K., Haug, J. L., Lust, J. A., Greipp, P. R. & Kumar, S. (2007) Leukemia 21, 1549-60
- Knapp, W., Majdic, O. & Strobl, H. (1993) Recent Results Cancer Res 131, 31-40.
- Knapp, W., Strobl, H. & Majdic, O. (1994) Cytometry 18, 187-98.
- Knapp, W., Strobl, H., Scheinecker, C., Bello-Fernandez, C. & Majdic, O. (1995) Ann Hematol 70, 281-96.
- Konikova, E., Glasova, M., Kusenda, J. & Babusikova, O. (1998) Neoplasma 45, 282-91.
- Lanza, F., Latorraca, A., Moretti, S., Castagnari, B., Ferrari, L. & Castoldi, G. (1997) Cytometry 30, 134-44.
- Millard, I., Degrave, E., Philippe, M. & Gala, J. L. (1998) Clin Chem 44, 2320-30.
- Nakase, K., Sartor, M. & Bradstock (1998) Cytometry 34, 198-202.
- Pascale, F., Contreras, V., Bonneau, M., Courbet, A., Chilmonczyk, S., Bevilacqua, C., Epardaud, M., Niborski, V., Riffault, S., Balazuc, A. M., Foulon, E., Guzylack-Piriou, L., Riteau, B., Hope, J., Bertho, N., Charley, B. & Schwartz-Cornil, I. (2008) J Immunol 180, 5963-72
- Pickl, W. F., Majdic, O., Kohl, P., Stockl, J., Riedl, E., Scheinecker, C., Bello-Fernandez, C. & Knapp, W. (1996) J Immunol 157, 3850-9.
- Riera-Sans, L., & Behrens, A. (2007) J Immunol 178, 5690-700
- Roberts, J. L., Lengi, A., Brown, S. M., Chen, M., Zhou, Y. J., O'Shea, J. J. & Buckley, R. H. (2004) Blood 103, 2009-18
- Sargent, R. L., Craig, F. E. & Swerdlow, S. H. (2009) Int J Clin Exp Pathol 2, 574-82
- Scheinecker, C., Strobl, H., Fritsch, G., Csmarits, B., Krieger, O., Majdic, O. & Knapp, W. (1995) Blood 86, 4115-23.
- Sedlmayr, P., Grosshaupt, B. & Muntean, W. (1996) Cytometry 23, 284-9.
- Strobl, H. & Knapp, W. (2004) J Biol Regul Homeost Agents 18, 335-9.
- Strobl, H., Scheinecker, C., Csmarits, B., Majdic, O. & Knapp, W. (1995) Br J Haematol 90, 774-82.
- Strobl, H., Scheinecker, C., Riedl, E., Csmarits, B., Bello-Fernandez, C., Pickl, W. F., Majdic, O. & Knapp, W. (1998) J Immunol 161, 740-8.
- Strobl, H., Takimoto, M., Majdic, O., Fritsch, G., Scheinecker, C., Hocker, P. & Knapp, W. (1993) Blood 82, 2069-78.
- Wang, X., Chang, X., Facchinetti, V., Zhuang, Y. & Su, B. (2009) J Immunol 182, 3597-608
Warranty
The products sold hereunder are warranted only to conform to the quantity and contents stated on the label at the time of delivery to the customer. There are no warranties, expressed or implied, that extend beyond the description on the label of the product. Exalpha`s sole liability is limited to either replacement of the products or refund of the purchase price. Exalpha is not liable for property damage, personal injury, or economic loss caused by the product.
Safety Datasheet(s) for this product:
| EA_SDS-GAS-002_V1 |
| EA_Fix and Perm SDS_JS07-05-19_V1 |
| EA_SDS GAS-002 with Sodium Azide |
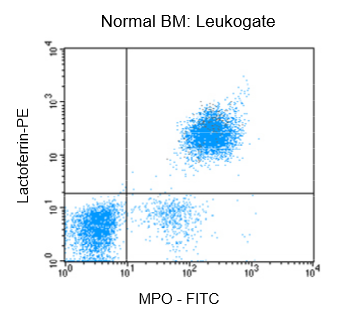
Figure 1: Flow cytrometric analysis of normal bone marrow (BM) after fixation and pemeabilization using GAS-002, followed by immunostaining for lactoferrin and MPO-C2.
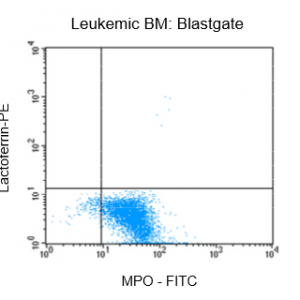
Figure 2: Flow cytrometric analysis of bone marrow (BM) from a leukemia patient after fixation and pemeabilization using GAS-002, followed by immunostaining for lactoferrin and MPO-C2.
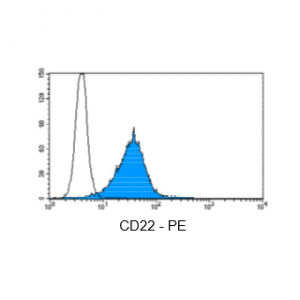
Figure 3: Immunostaining with Nordic-MUbio CD22-PE conjugate of undifferentiated leukemia cells of B-ALL type.
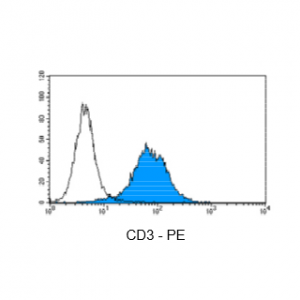
Figure 4: Immunostaining with Nordic-MUbio CD3-PE conjugate of surface CD3-negative undifferentiated leukemia cells of T-ALL type.
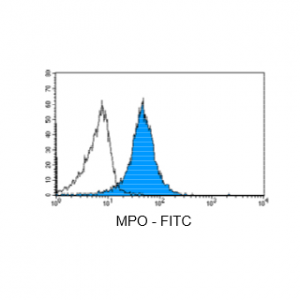
Figure 5: Immunostaining with Nordic-MUbio anti MPO-FITC conjugate of undifferentiated leukemia cells of AML type.
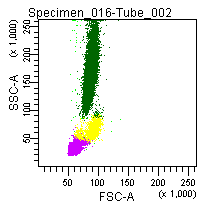
Figure 6: Forward and side scatter pattern of a normal blood sample treated with GAS-002.
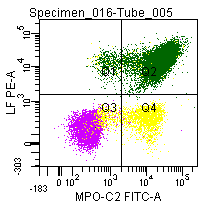
Figure 7: Double labeling of a normal blood sample treated with GAS-002, and immunostained for Lactoferrin (PE) and MPO-C2 (FITC).
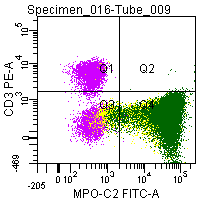
Figure 8; Double labeling of a normal blood sample treated with GAS-002, and immunostained for CD3 (PE) and MPO-C2 (FITC).
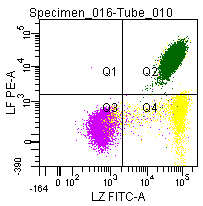
Figure 9: Double labeling of a normal blood sample treated with GAS-002, and immunostained for Lactoferrin (PE) and Lysozyme (FITC).
